
All elements can be represented in this fashion. Therefore, there are various non-equivalent definitions of atomic radius.\right] 5s^2\). Explanation: The atomic number of manganese is 25 and as such its electron configuration is.
#Ca element configuration free#
However, this assumes the atom to exhibit a spherical shape, which is only obeyed for atoms in vacuum or free space. The atomic radius of a chemical element is a measure of the distance out to which the electron cloud extends from the nucleus. Under the orbital approximation, we let each electron occupy an orbital, which can be solved by a single wavefunction. It must be noted, atoms lack a well-defined outer boundary. The electron configuration is the standard notation used to describe the electronic structure of an atom. The atomic radius of Calcium atom is 176pm (covalent radius). The electronic configuration of cations is assigned by removing electrons first in the outermost p orbital, followed by the s orbital and finally the d orbitals (if any more electrons need to be removed). Li Name of Element : Lithium Atomic Weight : 6. He Name of Element : Helium Atomic Weight : 4.0 Atomic Number : 2 Group : Nobel Gases Electron Configuration: 1s2 Go to the Top of the page. Note that, each element may contain more isotopes, therefore this resulting atomic mass is calculated from naturally-occuring isotopes and their abundance. How can I draw electronic configuration of calcium in a shell. Atomic Number : 1 Group : Non-Metals Electron Configuration: 1s1 Go to the Top of the page. It is the fifth most abundant element in Earth's crust. Its physical and chemical properties are most similar to its heavier homologues strontium and barium.

As an alkaline earth metal, calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. The atomic mass is carried by the atomic nucleus, which occupies only about 10 -12 of the total volume of the atom or less, but it contains all the positive charge and at least 99.95% of the total mass of the atom. Calcium is a chemical element with the symbol Ca and atomic number 20. The atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to a certain specific isotope of an element.

The reason for this is that the energy level of.
#Ca element configuration how to#
Mass numbers of typical isotopes of Calcium are 40 42 43 44 46. Valence electrons are the electrons in the outermost shell, or energy level, of an atom. Note that in the electron configuration of both K and Ca, the 4s orbital is filled before the 3d orbital. Calcium Electron Configuration Wayne Breslyn 628K subscribers 881 152K views 9 years ago A step-by-step description of how to write the electron configuration for Calcium (Ca). Isotopes are nuclides that have the same atomic number and are therefore the same element, but differ in the number of neutrons. The difference between the neutron number and the atomic number is known as the neutron excess: D = N – Z = A – 2Z.įor stable elements, there is usually a variety of stable isotopes. A well as beryllium and aluminium, and unlike the alkaline metals, it doesn’t cause skin-burns. The metal is trimorphic, harder than sodium, but softer than aluminium. Correctly gives the electron arrangements of two ions OR two atoms Shows. Neutron number plus atomic number equals atomic mass number: N+Z=A. The chemical element Calcium (Ca), atomic number 20, is the fifth element and the third most abundant metal in the earth’s crust. Therefore Ca and Cl ions now both have the same electron configuration of 2,8,8. The total number of neutrons in the nucleus of an atom is called the neutron number of the atom and is given the symbol N.
#Ca element configuration full#
The total electrical charge of the nucleus is therefore +Ze, where e (elementary charge) equals to 1,602 x 10 -19 coulombs. Calcium has 20 electrons so the full electronic configuration is: The shorthand version is Ar 4s2 since argon is the nearest preceding noble gas to calcium. First you should write their normal electron configuration and then when you remove electrons you have to take them from the outermost shell.
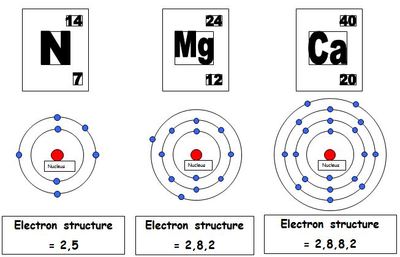
Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z. The electron configurations for Cationsare also made based on the number of electrons but there is a slight difference in the way they are configured. Atomic Number – Protons, Electrons and Neutrons in CalciumĬalcium is a chemical element with atomic number 20 which means there are 20 protons in its nucleus.


 0 kommentar(er)
0 kommentar(er)
